Chromatography- Food Colouring Experiment ( Individual Work)
Experiment Process:
Learning Points:
- Locating Agents can be used to convert colourless chemicals into coloured, such that it is possible to separate a mixture of colourless chemicals by chromatography
- Factors affecting the Rf of a chemical:
- Temperature: When temperature is higher, the dye is more soluble, hence it will travel higher up.
- Paper: When the paper changes, it may absorb the dye better, hence the rf value is lowered.
- solvent : type of solvent will affect the distance travelled by the dye, rf value higher/lower ( depending on the the amount of chemicals it dissolves
- Uses of Rf value:
- Identify the individual components in dye
- estimate/ predict the element.
Chromatography as a SYSTEM:
All the components must be present in order for the chromatography to work. It is made up of different parts with different functions to serve one common purpose -> identify and separate the mixture.
Principle: More Soluble -> travel farther than less soluble
--> difference in solubility ; enables separation
 |
| Chromatography almost done! |
 |
| Simple Distillation Setup |
*sorry for the slanted pictures
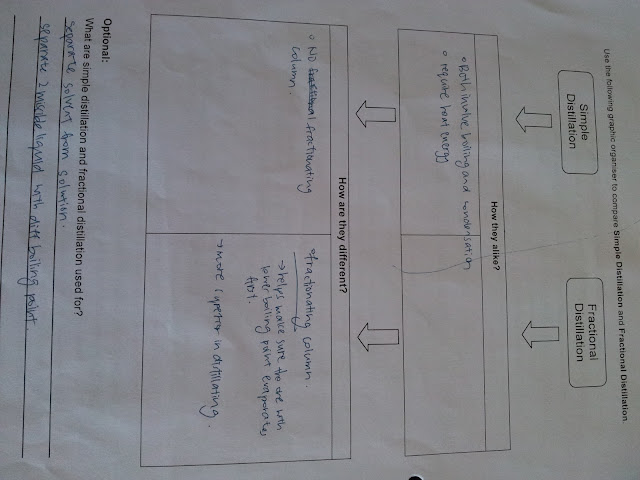
Fractional Distillation is Optional. Additional Reading
helpful link: http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/oneearth/fuelsrev1.shtml
Reflections:
As a hardcore CSI fan, it was really cool to be able to try hands on on Chromatography. I have seen it being done on the show countless times, and im finally able to understand and explain the concepts and uses behind it. The end product of my Chromatography Experiment turned out really well. ( refer to picture above). I could clearly see the components of the food colouring and i really liked the bands of colours on it that made up the strip dissolved by the solvent.
Although we could not try doing the Distillation experiment by ourselves, but it was still really interesting to see the coca cola being boiled and how it evaporates and condenses to have water as the distillate. In primary school, we used to have a simplified version of simple distillation ( just imagine how simple it was). And now, looking at the original simple distillation set-up, its really different!
All in all, this was an enriching and fun lab lessons :>
Extra notes found online. 28/1/13







No comments:
Post a Comment