Group Activity- Are you good enough for it ?
Question : How do you separate mixture of iron filings , iodine ( sublime when heated) , copper Sulfate (ll) , Calcium Sulfate , Ammonium chloride ( sublimes when heated ) and Lead (ll) bromide
suggested answer from mr foo's slides:
Element, Compound & Mixture
(Answer Techniques)
 |
| Nitrogen Atoms |
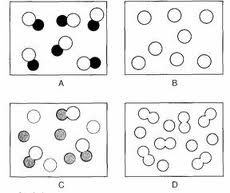
Element:
- This diagram shows a element
- REASON: (type of element) atoms combined to form diatomic molecules.
Mixture:
Mixture of elements:
____ and ___ are not chemically combined
Mixture of molecules:
two elements are in the form of diatomic molecules but are not chemically combined with one another
Mixture of 2 different compounds made up of the same elements:
same constituent elements in different ratio.
Mixture of 2 different compounds :
two different compounds that are not chemically combined with one another
Compound:
( pure) compound:
two different types of element chemically joined together in a fixed ratio.
*pure compound: A pure compound is a substance composed of a specific molecule or formula unit composed of 2 or more elements that cannot be broken down physically.
Separation Technique Assignment 1 for test !



No comments:
Post a Comment